


Once the magma erupts, it comes out to the surface, forming lava that flows, depositing ashes. This is mainly present in Earth’s upper mantle. The formation of volcanic mountains takes place from the surface eruption of magma.
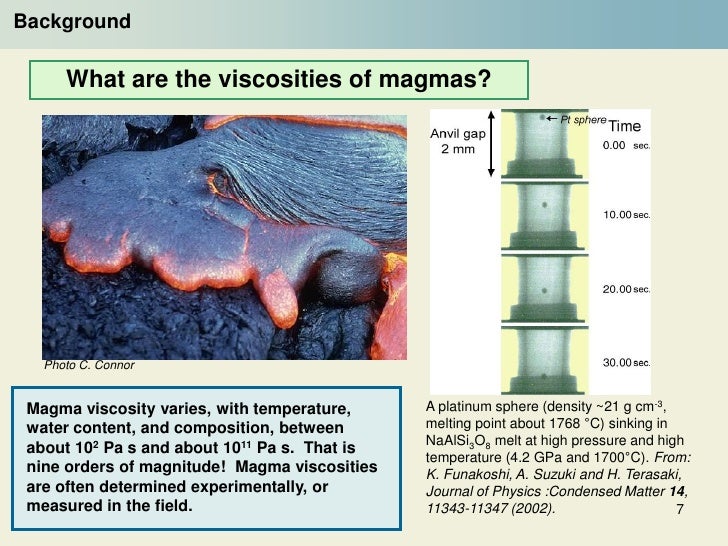
This is a potentially active volcano that is located southwest of British Columbia. One of the volcanic mountain examples is Mount Garibaldi. In other words, a volcano can be defined as an opening in the crust of a planetary-mass object from which hot lava, volcanic ash, and gasses come out via a magma chamber under the surface. In other words, a volcano eruption can be seen as similar to that of opening a bottle of soft drink, where the pressurized gasses spew outwards. One of the major reasons behind the volcanic eruption is the pressure that is created due to the dissolved gasses. Trace elements: The remaining elements (Minor elements: TiO2, MnO, H2O and P2O5 usually make up between 0.1 and 1%.Also, Na2O (2.5 - 4%) and K2O (0.5 - 5%) tend to increase with SiO2 content. Major elements: Oxides that account for >98% of the chemical composition of all igneous rocks, including: SiO2 (35 - 75%), Al2O3 (12 - 18%), FeO+Fe2O3 +MgO+CaO (20 - 30%).Most contain silicate minerals (in which Si, Al, Mg, Fe, Ca, Na and K combine with oxygen) and some minor oxide minerals. Granites (containing around >15% quartz, and more alkali feldspar than plagioclase feldspar) form >95 % of all igneous plutonic rocks, while basalts form >98 % of all volcanic rocks.Īs magma erupted at the surface is difficult to sample, the solid rocks that form after cooling provide us with the most information on the chemistry of magmas. The most common igneous rocks by far are granites and basalts. In some lava lakes, gases come out as invisible, colourless and odourless gas bursts and may kill many people and animals. Other gases that bubble out at the surface include nitrogen, oxygen, argon, carbon dioxide, boron, carbon monoxide, methane, hydrogen, nitric acid, hydrochloric acid, hydrofluoric acid, sulfuric acid, sulfur dioxide and sulfur trioxide. Some 99% of all gases in lavas are water vapour. Many lavas are vesicular (have cavities), indicating former gas bubbles which escaped from within the magma as it erupted at the surface. Conversely, silica-rich melts favour the crystallisation of silicates such as feldspars, micas and quartz (i.e. The silicate structures that develop favour the crystallisation of minerals of the olivine and pyroxene groups (i.e. Many melts are rich in basic oxides such as MgO and FeO and relatively low in silica. Therefore, a water-rich magma is more fluid than a dry magma of the same composition. Rises in temperature and in water content break down the structure of magmas and decrease their viscosity. Although silica-rich magmas have lower temperatures than silica-poor magmas, a high degree structure gives them higher viscosity. The viscosity of a silicate melt (liquid magma) is controlled by its degree of structure, with increased structure in magmas correlating strongly with increased viscosity. For example, syrup is more viscous than water. Viscosity is the resistance of a fluid to motion. It ranges from 2.4 g/cm3 (for silica-rich lavas) to 2.9 g/cm3 (for silica-poor lavas). The density of chilled magma (volcanic glass) is usually measured. Komatiitic lavas (very rich in magnesium and low in silica) probably erupted at 1300° C - 1400° C and mostly date from the Archean Era when the earth was hotter. Less silica-rich lavas generally come out at 1000° C - 1230° C while silica-rich lavas are cooler at 750° C - 900° C. The temperature of some lavas can be measured in the field using a thermocouple providing the eruption is not violent (as in lava flows or lakes). for air temperature, distance, and elevation). It can be done at a distance using an optical pyrometer (glowing filament) but this needs many corrections (e.g. The temperature of a magma is hard to measure. The properties of magmas include temperature, density, viscosity, gas content and abundance. Some solidify within the earth (plutonic or subvolcanic rocks). Magmas consolidate at the surface as lava flows (volcanic), or fall as lithic (rock), crystal and/or glass fragments (pyroclastic).


 0 kommentar(er)
0 kommentar(er)
