
#HEISENBERG PRINCIPLE OF OBSERVATION FULL#
In the published 1927 paper, Heisenberg originally concluded that the uncertainty principle was Δ pΔ q ≈ h using the full Planck constant. Introduced first in 1927 by German physicist Werner Heisenberg, the uncertainty principle states that the more precisely the position of some particle is determined, the less precisely its momentum can be predicted from initial conditions, and vice versa. It states the we cannot precisely know both the. This phenomenon is called the Heisenberg uncertainty principle. The uncertainty principle implies that it is, in general, not possible to predict the value of both paired variables’ quantities with arbitrary certainty beyond certain limit, in which a trade-off (frequency-position trade-off) between both appears, even if all initial conditions are specified and known. The link between reality and observation is based on what has been called the Copenhagen Interpretation of quantum mechanics because it was proposed by Niels Bohr, Werner Heisenberg, and other physicists working in that city. Such paired-variables are, therefore, known as complementary variables or canonically conjugate variables and, depending on interpretation, the uncertainty-principle limits to what extent such conjugate properties maintain their approximate meaning, as the mathematical framework of quantum physics does not support the notion of simultaneously well-defined conjugate properties expressed by a single value. In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy the values for certain related pairs of physical quantities of a particle, such as position, x, and momentum, p, can be predicted from initial conditions appearing a trade-off between them. Therefore, a meaningful answer to the Heisenberg uncertainty principle is present. Uncertainty principle of Heisenberg, 1927. How Quantum Suicide Works Prev NEXT By: Josh Clark Heisenberg's Uncertainty Principle Werner Heisenberg AFP/ Getty Images One of the biggest problems with quantum experiments is the seemingly unavoidable tendency of humans to influence the situation and velocity of small particles. That is, for a special observation, the uncertainty may essentially an.
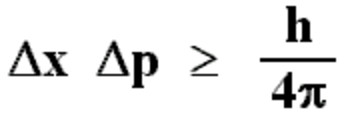
To precisely measure a wave's energy would take an infinite amount of time while measuring a wave's exact instance in space would require to be collapsed onto a single moment which would have indefinite energy.Canonical commutation rule for position q and momentum p variables of a particle, 1927.

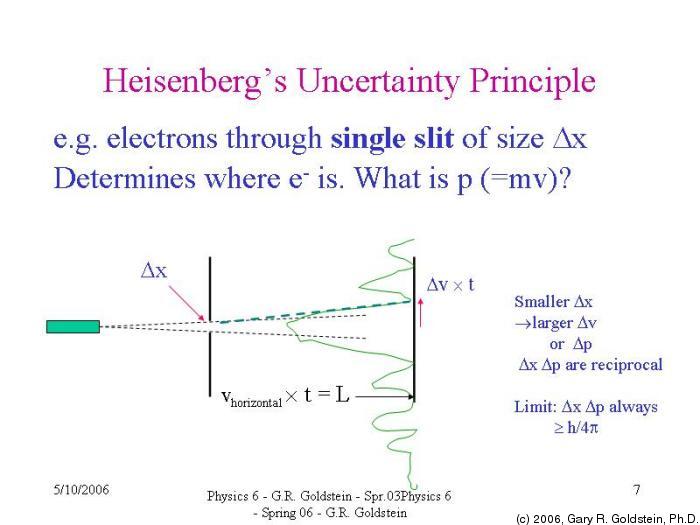
You could do the same thought experiment with energy and time. Similarly, a wave with a perfectly measurable momentum has a wavelength that oscillates over all space infinitely and therefore has an indefinite position. It is instructive for each of us to know that there is such a thing as the Observer Effect, and it helps us realize that we have the opportunity as leaders to know and act upon it. Very roughly, it states that if we know everything about where a particle is located (the uncertainty of position is small), we know nothing about its momentum (the uncertainty of momentum is large), and vice versa. He considered trying to measure the position of an electron with a gamma ray microscope. Heisenberg’s uncertainty principle is a key principle in quantum mechanics. A wave that has a perfectly measurable position is collapsed onto a single point with an indefinite wavelength and therefore indefinite momentum according to de Broglie's equation. Heisenberg conducted a thought experiment as well. Let's consider if quantum variables could be measured exactly.


 0 kommentar(er)
0 kommentar(er)
